PDF) INVESTIGATIONAL NEW DRUG APPLICATION (IND) (Title 21, Code of Federal Regulations (CFR) Part 312 | Myriam tGozalez Ruiz - Academia.edu

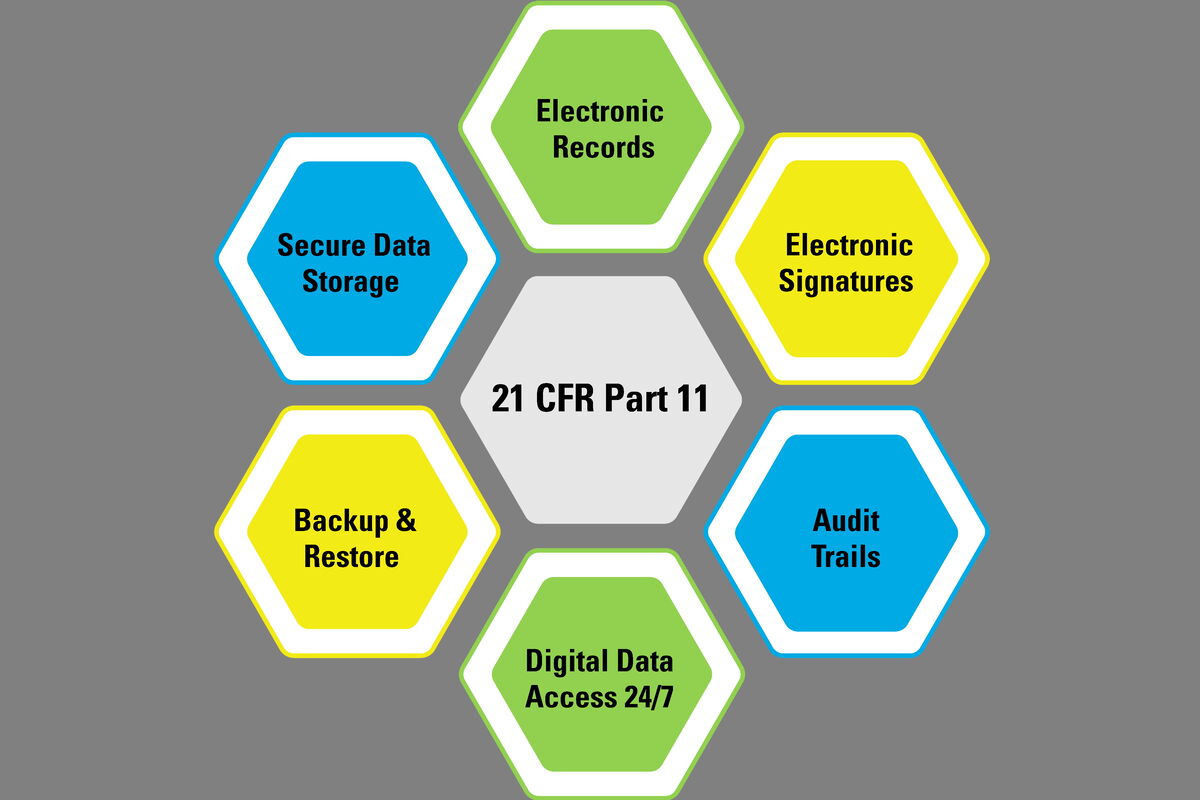
GMP Regulation Handbook: Electronic Signatures, 21 CFR Part 11 | ISPE | International Society for Pharmaceutical Engineering
FDA 21 CFR Part 11 Training, Regulations, and Best Practices - GxP Training : Certified Online Courses for Life Sciences
![Attachment 1. Excerpts from Federal Food and Drug Administration regulations [Code of Federal Regulations] [Title 21, Volume 2] Attachment 1. Excerpts from Federal Food and Drug Administration regulations [Code of Federal Regulations] [Title 21, Volume 2]](https://forloveofwater.org/wp-content/uploads/2017/07/Attachment-1-1-pdf.jpg)